What is Matter?
- Anything that has mass and occupies space
Chemist view .. matter is what chemical substances are composed of
Physical Properties can be observed/measured without changing the composition of matter. They are used to describe matter.
- Color
- Odor
- Luster (how shiny)
- Hardness (resistance to scratching)
- Malleability (ability to be pounded into thin sheets)
- Buoyancy (ability to float in a fluid)
- Viscosity (resistance to flow)
- Elasticity (ability to be stretched and return to original size)
- Density (relationship between mass and volume)
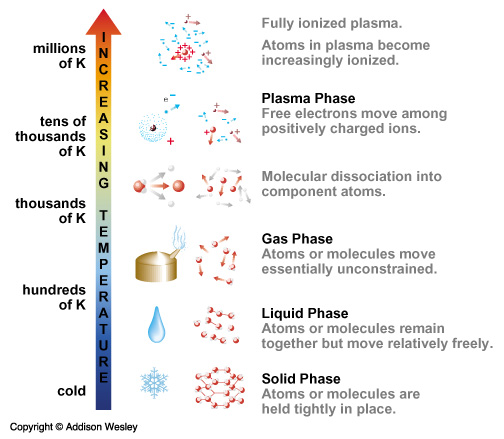
- melting/boiling/freezing point - Solid, Liquid, or Gas
Ways matter Physically Change
- Cutting wood
- Melting ice cream
- Boiling water
- Freezing juice for popsicles
- Cracking an egg
* A Physical Change alters the substance without changing the composition of the substance*
Chemical Properties describes matters "potential" to undergo some chemical change or reaction. Can only be observed when the identity of the subject is changed.
- Flammability
- Reactivity
- Toxicity
- Oxidation
Ways matter Chemically Change
- Rusting nail
- Burning wood
- Explosion of dynamite
- Rotting food
- Baking a cake
- Cooking an egg
* A Chemical Change alters the composition of the substance, a new substance is formed*
Signs of a Chemical Change
- Gas bubbles form (possible new odor)
- Light, heat, or sound is given off (or absorbed)
- Change in color
- Formation of solid matter (precipitate) in liquid
- Decomposition of matter


0 comments:
Post a Comment